From Eli Lilly abandoning evacetrapib in the final stages of trials to the surprising placebo effect, this week’s Research Ethics Roundup shines the spotlight on clinical trials that could shape the future of research.
 Gene Editing Record Smashed in Pigs: A team from Harvard Medical School, led by geneticist George Church, has used CRISPR technology to modify more than 20 genes in pig embryo and deactivate more than 60 viruses that could cause disease in human transplant recipients. Church’s group hopes that this will pave the way for nonhuman organ donation.
Gene Editing Record Smashed in Pigs: A team from Harvard Medical School, led by geneticist George Church, has used CRISPR technology to modify more than 20 genes in pig embryo and deactivate more than 60 viruses that could cause disease in human transplant recipients. Church’s group hopes that this will pave the way for nonhuman organ donation.
Eli Lilly Abandons Heart Disease Drug in Final Stages of Trials: Eli Lilly announced it is ending the development of evacetrapib, a drug intended to prevent heart attacks and strokes. The study was in the phase three stage of clinical trials, and Lily reached the decision to stop the study after its safety monitoring committee found that it was unlikely to be effective in reducing the risk of cardiovascular problems and death.
Strong Placebo Response Thwarts Painkiller Trials: An extensive review of United States (US) clinical trial data on painkillers has found that subjects receiving placebo treatments have reported improvement at almost the same rate as those subjects on the experimental drug; while the rate of placebo responses has risen over the past twenty years, the rate of drug responses has remained the same. However, this rise in placebo responses cannot be found in similar trials taking place outside the US.
British Nurse’s Re-Hospitalization, Reports of Blindness and Other ‘Post-Ebola Syndrome’ Complications Haunt Survivors: Many subjects treated for Ebola with experimental drugs during the 2014 crisis have reported serious effects, including vision problems, chronic fatigue, and liver problems. Doctors are unsure if these medical issues are directly related to the virus, or if they are complications from the treatment survivors received. However, doctors are confident that if the virus is still present in these individuals, the risk of transmission is incredibly low.
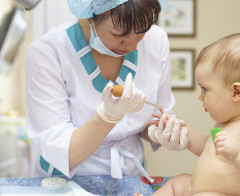
Many Babies Exposed to Unnecessary Pain Research: A research team has concluded that babies often suffer from unnecessary pain in clinical studies, finding that nearly two-thirds of the studies they reviewed involved either painful procedures or groups of children who were not given pain relief. The report’s authors state that some of the studies they reviewed potentially breach international standards of ethical research. Infants experience pain more powerfully and feel physiological changes more acutely than adults.

No comments! Be the first commenter?