As we mark PRIM&R’s 50th anniversary this year, it’s a moment to reflect on the rich history of our certification programs, the Certified IRB Professional (CIP®) and the Certified Professional in IACUC Administration (CPIA®). Embark with us on a journey through our thriving community’s certification history.
CIP Program History
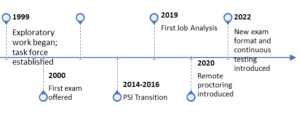
The CIP program was founded in 1999 under the direction of ARENA leadership. The original task force was chaired by Gary Chadwick, PharmD, MPH, CIP, who, at the time, was also running the Human Research Protection Program (HRPP) at the University of Rochester School of Medicine.
During the mid-1990s, a series of government shutdowns of research programs occurred in response to the deaths of healthy research subjects. Throughout this challenging period, the PRIM&R/ARENA community strengthened, engaging in extensive discussions regarding the potential implementation of new federal mandates for training and other requirements for researchers and research oversight personnel. There was apprehension that these proposed measures would increase administrative burdens without enhancing subject protections or safety.
Dr. Chadwick spearheaded a task force to investigate the possibility of establishing a voluntary certification program for HRPP/IRB oversight professionals. The aim was for the federal government to acknowledge the community’s commitment to self-regulation, thereby avoiding the imposition of its own certification or training mandates.
This volunteer group collaborated with PTC, a New York-based credentialing company, to establish the CIP program, developing and validating exam items (questions) and crafting eligibility and recertification guidelines.
The first exam was offered in-person at the December 2000 PRIM&R/ARENA annual conference. From 2001 to 2005, exams were available in the fall at the annual conference and at testing centers in the spring. By 2020, PRIM&R began offering a live remote online proctoring option, allowing candidates to take exams from home. In 2022, we introduced a continuous testing model, replacing semiannual testing windows with 90-day eligibility periods for candidates to test.
PRIM&R’s Executive Director, Ivy Tillman, EdD, CCRC, CIP, reflected on the value of obtaining a CIP credential on her career. Earning a CIP “changed everything,” she said, noting that she is “a huge advocate for certifications.”
“It has allowed me to have credibility not only in academic settings, but also the industry,” Dr. Tillman said. “It broadened my understanding of the field, but it’s also challenged me. It isn’t just obtaining the certification, it’s maintaining it. It’s challenged me to make sure I stay informed, educated, and involved, particularly in human subjects research.”
Dr. Tillman emphasized the dual significance of the CIP credential, extending its influence beyond academia to enhance credibility in diverse professional settings.
CPIA Program History
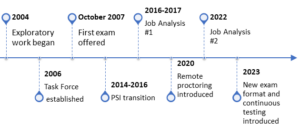
In 2004, PRIM&R assembled a committee comprising leaders in the IACUC administration field to establish the CPIA program. A grant from AALAS helped offset start-up costs, and from 2006 to 2017, an AALAS representative served on the CPIA Council, offering expertise on testing administration and exam development. Drawing inspiration from the CIP program, the original task force shaped the CPIA exam format and eligibility guidelines. They also looked to AALAS’ certification programs as a benchmark, given the familiarity of much of the target audience with its operations.
In 2016, the CPIA Council commenced its job analysis, managed by AALAS, coinciding with the transition to AMP, a Kansas-based testing administrator that focused on small and mid-sized clients and had experience in working with regulatory healthcare programs. Although AMP didn’t oversee the project, they provided feedback on the survey, fostering a trust-building opportunity with CPIA Council members.
In 2020, the introduction of live remote online proctoring allowed candidates to take exams from their personal computers. Continuous testing and instant scoring were implemented in 2023. A job analysis facilitated the modernization of the exam format from 250 multiple-choice questions to 135. Revised content outlines and eligibility guidelines were put into effect, offering candidates a 90-day testing window and delivering scores within an hour of testing.
As we celebrate the milestones of PRIM&R’s certification programs, we invite you to embrace the opportunity to distinguish yourself in the field of research ethics. Whether you are pursuing initial certification or renewing your credentials, PRIM&R’s programs offer a prestigious recognition of expertise and commitment. Don’t miss out on the chance to elevate your career—certify or recertify today and join us in shaping the future of ethical research. If you have any questions about certification, reach out to our certification team.


No comments! Be the first commenter?